The Nitrogen Cycle
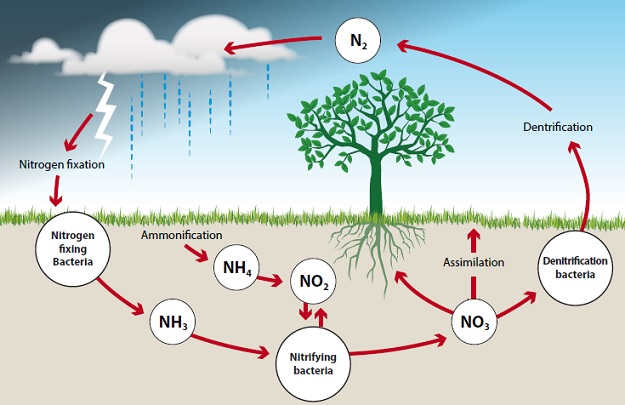
The nitrogen cycle illustrates the relationship between different forms of nitrogen in the soil, water, air and living organisms. It is considered to be a cycle since nitrogen moves around from place to place in different forms but is always present. The main phases of the cycle are shown in the following diagram and are discussed below:
1. Ammonification
Here, organic nitrogen from dead plants and soil organisms is converted to ammonium (NH4+) by mineralisation. Mineralisation is the decomposition of organic matter to NH4+ which is a usable nutrient ion. This is an inorganic form of nitrogen (plants cannot absorb organic nitrogen). Many different soil organisms are involved in the ammonification process including bacteria and fungi. These soil organisms break down organic matter using the carbon and energy produced, but nitrogen is released.
2. Nitrification
This is the oxidation of ammonium ions from mineralisation to nitrate anions via chemoautotrophic bacteria. These are organisms that aid in the nitrification process i.e. nitrosomonas and nitrobacter. They use energy obtained by oxidisation-reduction reactions in the conversion of NH4+ to NO2-, and use carbon from CO2 in the soil atmosphere. Nitrosomonas convert ammonium to nitrite and nitrobacter convert nitrite to nitrate. pH has an impact upon which part of the process can occur.
Nitrite is poisonous to plants if allowed to accumulate, which fortunately rarely occurs.
Optimum conditions for nitrification:
Adequate aeration
Optimum temperature 25 - 35oC
Adequate soil moisture
Adequate exchangeable bases - particularly Calcium (Ca)
NPK available
Requires a low C/N ratio
3. Denitrification
This refers to the change (via denitrifying bacteria) of nitrate to gaseous forms of nitrogen such as nitrogen gas (N2) or nitrous oxide (N2O). The process is rapid and large amounts of N2 are lost from the soil system and end up in the atmosphere. This typically happens when there is a lack of oxygen in the soil, for instance when the soil is too wet. As a soil dries out, denitrification ceases.
Conditions favouring denitrification:
Lack of adequate O2
Requires energy source of oxidisable organic matter for bacteria
Warm, slightly acidic soils.
4. Nitrogen Fixation
During nitrogen fixation nitrogen gas (N2) is converted back to ammonium (NH4+) by bacteria in soil or water, or by bacteria which have a symbiotic relationship with plants, such as rhizobium bacteria on the roots of legumes.
5. Immobilisation
Immobilisation can be viewed as the opposite of mineralisation. It refers to the use or re-use of soluble nitrogen (NH4+ and NO3-) by plants or microbes. The nitrogen is used to form new organic compounds to be cycled again through mineralisation and nitrification. This takes place in conjunction with ammonification.
Immobilisation of organic matter is the opposite process to mineralisation. In immobilisation, mineralised nutrients are incorporated into organic molecules within living cells. The process of immobilisation is very important because it relocates mineral nutrients into pools within the soil that have a relatively rapid turnover time, making them available to plants and preventing their loss by leaching. Plants are generally not efficient at competing with microorganisms for mineral nutrients in soil.
6. Ammonium Fixation
This is the process by which certain clays bond NH4+ so tightly between mineral lattices that it is not exchangeable, or available to plants for uptake. It is common in vermiculite, montmorillonite, and iolite. It occurs only in small amounts.
STUDY OUR LAND MANAGEMENT COURSE TO BE A MUCH BETTER LAND MANAGER, FARMER or GARDENER -
Click for details